Visualizing Oxygen Dynamics in Plant Tissue
Jagadis Gupta Kapuganti
Biochemistry & Systems Biology, Department of Plant Sciences, University of Oxford, GB
In order to prevent pathogens from spreading, infected plants use a form of cell death - the so called hypersensitive response. In this study the widely used model sytem of Arabidopsis and Pseudomonaswas used for studying plant-pathogen interactions and disease development. The VisiSens™ oxygen imaging system was applied for closer investigation of oxygen distributions in infected leaves undergoing hypersensitive response. Results showed that there was no change in oxygen levels when plants where treated with control medium, while oxygen concentrations in leaves infected with Pseudomonas syringaestarted to decrease after six hours. Within 24 hours those leaves reached oxygen levels of 50 %.
Hypersensitive response (HR) is a form of cell death. It is a mechanism that plants use to prevent the spread of pathogens. One of the characteristics of HR is rapid cell death that takes place in the infected region of the host in order to prevent further spread of the pathogen.Pathogens secrete avirulent gene products which are coded by avr genes. These avrgene products interact with plant resitance gene products (Rgenes) and this interaction leads to the development of HR. Cells undergoing HR produce various free radicals such as hydrogen peroxide (H2O2) superoxide (O2-), nitric oxide (NO), peroxynitrite (ONOO-), and many intermediate free radicals. These compounds participate in signalling during HR development. Oxygen is vital for plant respiration. It acts as terminal electron acceptor in the plant´s mitochondrial electron transport chain. Oxygen production and consumption take place in the green leaves. Oxygen content and respiration play a role in production of various free radicals, and in plant metabolic reprogramming. Arabidopsisand Pseudomonasis a widely used modelsystem for studying plant-pathogen interaction and disease development. So far a lot of reasearch has been performed in this model to understand plant-pathogen interactions but almost nothing is known about the oxygen distributions in infected leaves undergoing HR. This information is very important for understanding plant disease development and resistance mechanisms. The virulent strain P. syringaepv. tomato DC3000 avr (Pst) produces toxins which induce chlorosis and lesions in the infected host tissue. In this study, we used the VisiSens oxygen imaging system to monitor oxygen distributions in infected leaves over longer time periods. The optical sensor foil was attached to the surface of the leaf and read out with the detector unit. The recorded images were used to quantify oxygen changes in the infected leaves.
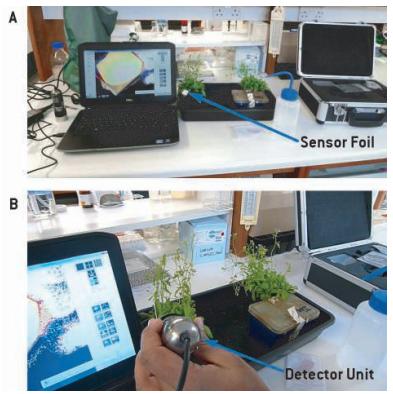
Fig. 1: Set-up for oxygen measurement on Arabidopsisleaves infected with Pseudomonas syringaepv. DC3000 (A). Reading the sensor response with the VisiSens detector unit (B).
Materials & Methods
An oxygen sensitive sensor foil (SF-RPSU4, PreSens) was placed on the investigated leaves and read out with the USB powered detector unit (VisiSens™, PreSens). Two point calibration of the sensor foil was performed by placing a drop of freshly prepared sodium dithionate (Na2S2O4) in the center of the sensor foil and an image was recorded,which was used for later computing of oxygen concentrations from ratio values, done with the VisiSens AnalytiCal 1 software. After recording the calibration value, the sensor foil was first sterilized by wiping with 70 % ethanol and wiped several times with autoclaved double distilled water. When the sensor foil had dried it was placedon the leaf. A drop of water was placed on the leaf in order to allow direct contact of sample surface and sensor foil. Pseudomonas syringaepv. DC3000 bacteria were grown over night in KB medium, then centrifuged at 3000 g for 5 minutes at 4 °C, and washed 3 times with 10 mM MgCl2. They were resuspended in MgCl2 and optical density was measured at 600 nm. The bacteria were diluted in 10 mM MgCl2 to 0.005 and then infiltrated into the abaxiel side of the leaf. After infiltration the sensor foil was placed on the leaf and the oxygen content recorded. Oxygen was measured at 0, 2, 4, 6, 16, and 24 hours.
Oxygen Levels in Infected Leaves
At present it is very important to know oxygen dynamics in plant tissues subjected to pathogen attack. The sensor gave information about oxygen distribution and dynamics during the infection process. It was possible to get quantified oxygen values, which are important for the interpretation of the data. Leaves infected with avirulent pathogens undergo various changes. Recognition of a pathogen or pathogen derived elicitors triggers oxidative bursts and this leads to the production of various free radicals. In order to fully understand ROS in pathogen infected plants oxygen changes are a key factor. For instance hypoxia induces nitric oxide production. The produced NO in turn reacts with superoxide and forms peroxinitrite (ONOO-), which is a very important compound inducing cell death. In these experiments it was found that there are no changes in oxygen levels when leaves are treated with 10 mM MgCl2 as a control (Fig. 3). In leaves infected with Pseudomonasoxygen levels started to decrease from hour six on (Fig. 2) . After 16 hours the oxygen concentration dropped to 70 % and reached 50 % after 24 hours (Fig. 4).

Fig. 2: Images of leaves infiltrated with Pseudomonas syringaepv. DC3000 taken with VisiSens™.
Fig. 3: Images taken of MgCl2(control) infiltrated leaves.

Fig. 4: Quantified oxygen contents (% air sat.) in Arabidopsisleaves infiltrated with Pseudomonas over time; oxygen content is above air saturation in MgCl2treated leaves, and slightly elevated in hours 16 and 24.
Decrease in oxygen content at later time points during infection can have several reasons: One possibility is that hypersensitive response shares many processes with senescence. Mitochondria may disintegrate during later stages of this development. Another possible reason is bacteria consuming oxygen and developing rapidly, while they compete with the leaves for oxygen. Third, the green leaf photosynthesis is affected, which leads to less oxygen production.
Conclusion
With the VisiSens™ oxygen imaging system it was possible to determine oxygen distributions and dynamics on infected plant leaves. The experiments gave first insight in temporal changes of oxygen distributions during disease development. Further experiments, using different virulent and avirulend pahtogens, and measuring oxygen in different transgenic plants infected with various pahtogens will give very valuable information about biochemical events during plant-pathogen interactions.