Checking Microvascular Perfusion in Mouse Kidney with the VisiSens™ System
Zoltán Varga
Cardiovascular Research Group, Department of Biochemistry, Universtiy of Szeged, Hungary
In this study we used a mouse model of renovascular hypertension known as the two-kidney, one clip model (2K1C). In this model one renal artery is constricted which ultimately results in an increase in circulating angiotensin II and a rise of blood pressure. The VisiSens system was used to monitor microvascular perfusion in the clipped kidney and make sure the kidney has decreased but still proper perfusion. The oxygen images created with the VisiSens system allowed evaluating the perfusion status of mouse kidneys before and after clipping. This way it was possible to determine animals with renal infarction, which had to be excluded from the experiments.
The mouse remains the animal of choice in transgenic experiments, creating a need for methods of evaluating the physiology of genetically modified animals. We have established and characterized a mouse model of renovascular hypertension known as the two-kidney, one clip model (2K1C). This model is characterized by the development of cardiovascular hypertrophy, which is an important field of pharmacological investigation. In the 2K1C model, one renal artery is constricted to chronically reduce renal perfusion, and the other kidney remains untouched. In this model the earliest phase of hypertension is marked by a rapid rise in plasma renin - an enyzme released in the kidney in response to low renal arterial pressure.This consequently increases the amount of circulating angiotensin II, which is responsible for a rise in blood pressure. The apporpriate size of the clip lumen needed to induce high blood pressure was determined to be 0.12 mm. Methodically it is important to check, whether the clipped kidney has decreased but still proper perfusion, since animals with renal infarction should be excluded from the experiments. Here we used the VisiSens™ oxygen imaging system for in vivo monitoring to check microvascular perfusion before and after performing the procedure on the renal artery

Fig. 1: . U-shaped stainless steel clips (3 x 2 x 1 mm size with a 2-mm-long cleft) used to constrict the renal artery. The width of the clip opening was 0.127 mm.

Fig. 2: Set-up for microvascular perfusion monitoring: Oxygen sensor foil placed on the mouse kidney; the VisiSens™ detector unit was fixed in a stand above the sensor foil for oxygen image recording (A). VisiSens™ detector unit fixed next to the operating microscope (B).
Materials & Methods
Male C57BL/6 mice (Charles River) with an approximate weight of 20 g were used at 4 to 5 weeks of age. They were maintained on tap water and a regular rodent chow ad
libitum. For clipping, U-shaped stainless steel clips were used (3 x 2 x 1 mm size with a 2-mm-long cleft). The width of the clip opening was 0.127 mm. Mice were anesthetized with sodium pentobarbital (50 mg/kg) which was maintained by isoflurane inhalation. The kidney was exposed through a small flank incision, externalized. For clipping, the renal artery of the left kidney was individualized over a short segment by blunt dissection, and a clip was placed close to the aorta. The kidney was then gently pushed back into the abdominal cavity, and was covered with the oxygen sensor foil (SF-RPSu4). Sham-operated animals were used as control. To have proper fixed position, we made a holder for the VisiSens™ detector unit DU01, which was applied first on the operation table (see Fig. 2 A) but later we fixed it right next to the operating microscope (see Fig. 2 B). With this set-up we were able to take oxygen images of the microvasculation of the kidney before and after clipping of the renal artery.
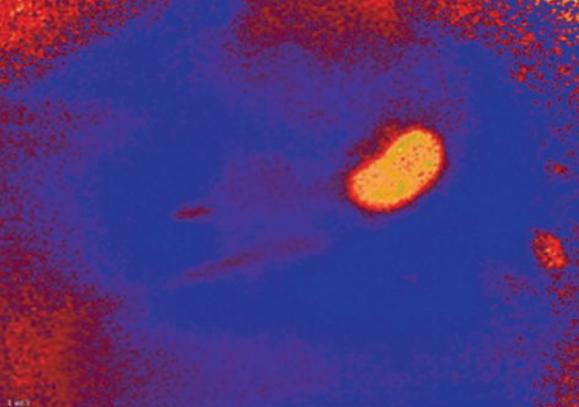
Fig. 3: Oxygen image taken before renal artery ligation. The image shows high oxygen levels in the properly perfused kidney tissue (indicated by yellow color).
Oxygen Imaging of Mouse Kidneys
With the VisiSens™ system we were able to take oxygen images of the treated mouse kidneys. The recorded picture in Fig. 3 shows microvascular perfusion before the clipping and renal artery constriction. After placment of the occluder on the artery a clear decrease in perfusion, showing up in lower oxygen concentrations in the kidney tissue, could be detected (Fig. 4). Monitoring microvascular perfusion in the clipped kidneys was important to check if the blood flow was not completely occluded. Fig. 5 shows the oxygen image of a totally occluded kidney. There the kidney tissue showed extremely low oxygen concentrations indicating that it was not perfused properly anymore. This animal had to be excluded from the experiment. Four weeks after clipping, two-kidney, one clip hypertensive mice exhibited blood pressure approximately 20 mmHg higher than their sham-operated controls.
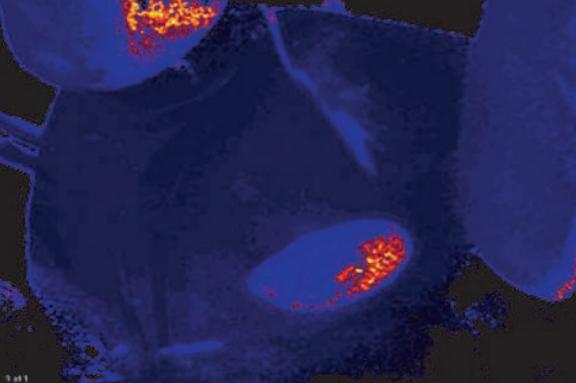
Fig. 4: Oxygen image of mouse kidney after placement of the occluder; slightly reduced oxygen levels in the tissue could be observed.
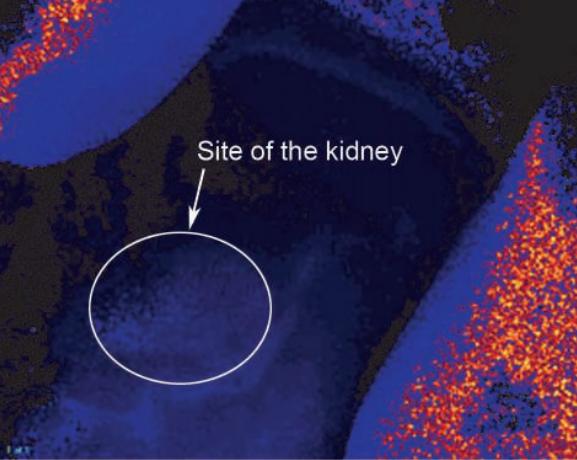
Fig. 5: Total occlusion of the mouse kidney; the tissue is not supplied with oxygen anymore, clearly showing in the oxygen image.
Conclusion
We found VisiSens™ to be applicablein vivofor analyzing the microcirculation in solid organs (kidney, liver, skin), however there are some limitations when using the sensor foil on irregularly shaped surfaces. The VisiSens™ AnalytiCal software is scientifically very impressive and user-friendly. The oxygen imaging system proved to be a very useful tool checking microcirculation and oxygen supply, and with it the condition of the organs of interest so decisions about continuing the experiment with the respective animal could be made.